공지사항
법령
[Chemlinked] Thailand Issues Notification on the Renewal of Cosmetics Notification Receipts
첨부파일
등록일 2022-08-09
조회수 6779
On June 22, 2022, the Thai Food and Drug Administration (Thai FDA) published a notification on the application for the renewal of cosmetic notification receipts. 1
The key points of the notification are summarized as follows:
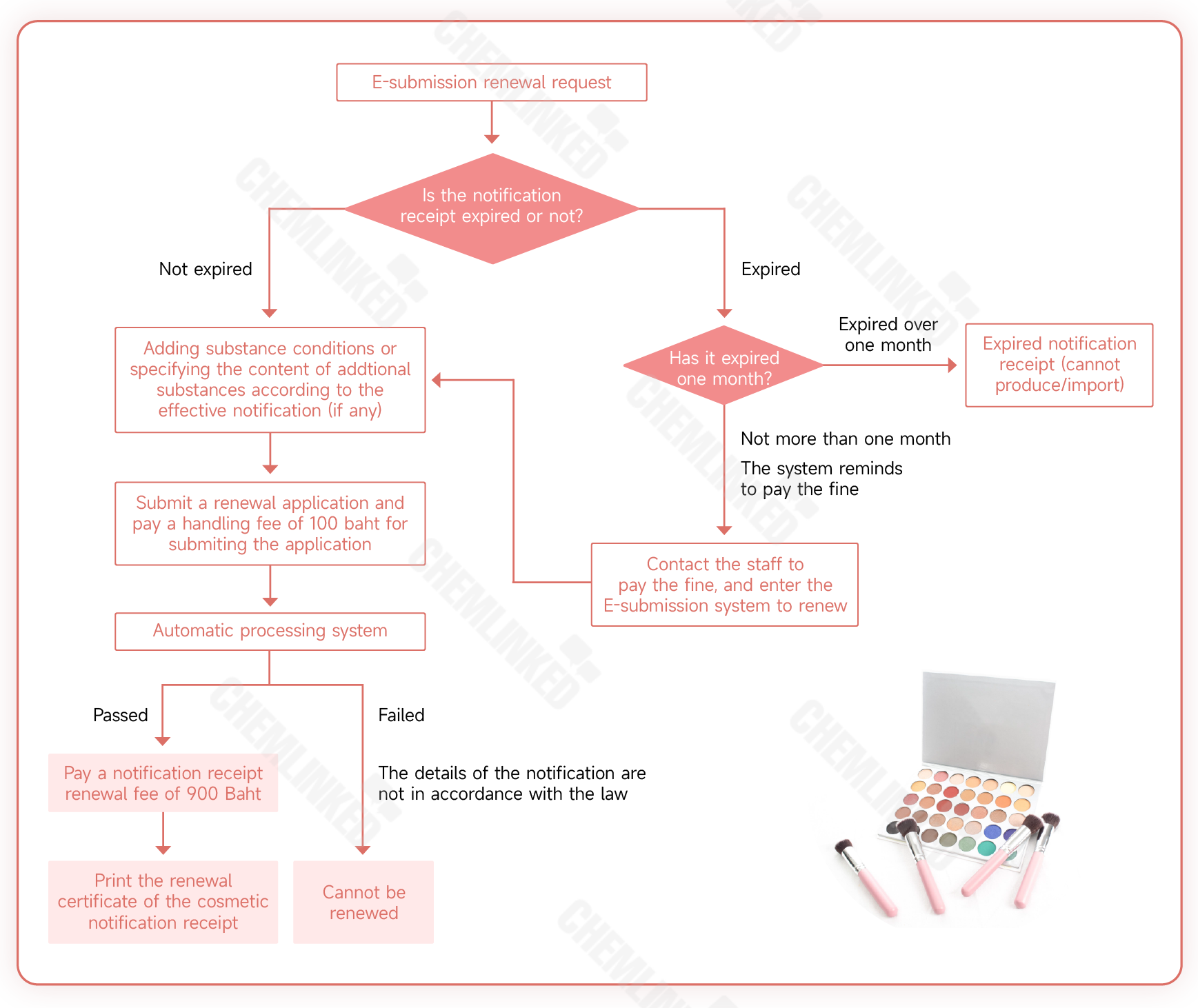
1. Pursuant to the Cosmetic Act B.E. 2558 (2015) and relevant notices from the Ministry of Public Health (MOPH), cosmetic manufacturers and importers shall obtain a notification receipt (cosmetic license) from the regulatory authority before undertaking the manufacture of cosmetics in or import of cosmetics to Thailand. The notification receipt remains valid for three years from the issue date and can be renewed.
2. Notifying parties planning to renew the notification receipt can, within 180 days before the expiry date, apply directly to the Thai FDA or the Provincial Health Office, or via the e-submission system. Then the notifying parties shall pay the renewal fee. Otherwise, the product license will lapse.
3. In the case of delayed renewal that the notification receipt has expired not more than one month, renewal application is still allowed but the reason for the delay shall be explained, and a fine shall be paid according to the overdue date.
4. The detailed renewal process is shown in the figure below.
Reference Links
[1] The Thai FDA’s Notice on Cosmetics Notification Receipt Renewal
출처 : Chemlinked








