공지/교육
법령
[유럽, EC Regulation 1223/2009] Annex III(배합한도), VI(자외선차단제) 개정 (비에이치티, 호모살레이트 등)
등록일 2022-11-17
조회수 8292
유럽 화장품 규정(EC No.1223/2009) 부속서 III, VI 개정 관련 안내드립니다.
세부 내용은 아래 사항을 참고해주시기 바랍니다.
출처 : Chemlinked
On November 11, 2022, the EU released the Commission Regulation (EU) 2022/2195, introducing new amendments to the use requirements for cosmetic ingredients in Regulation (EC) No 1223/2009 (Cosmetics Regulation). All amendments aim at the lists of restricted ingredients and permitted UV filters, based on the opinions from Scientific Committee on Consumer Safety (SCCS). 1
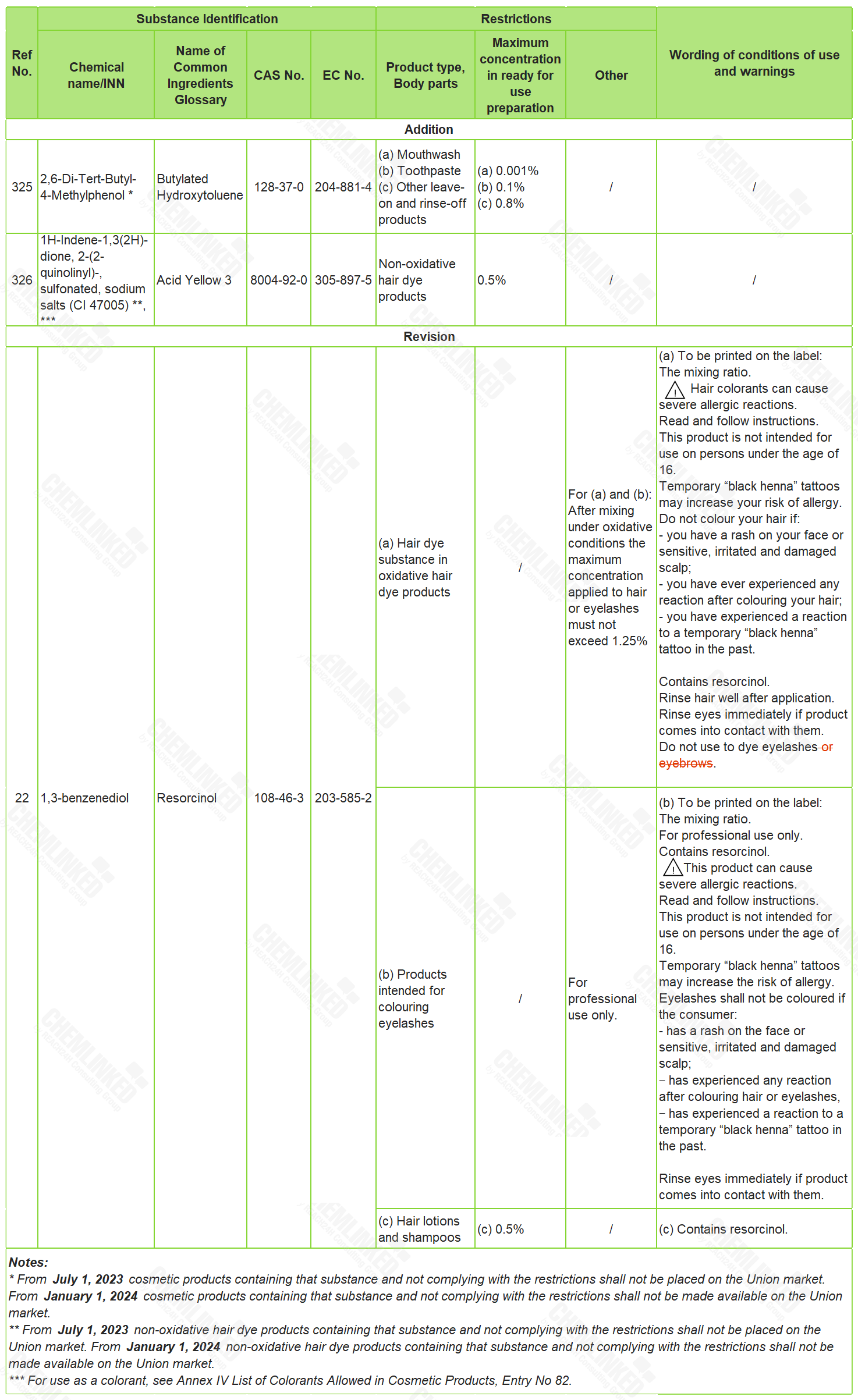
1. List of Restricted Ingredients: two added and one revised
Butylated Hydroxytoluene (CAS No 128-37-0) is currently not included in the ingredient lists in the Cosmetics Regulation, while Acid Yellow 3 (CAS No 8004-92-0) is an annexed colorant allowed for use in cosmetics without maximum concentration. In 2021, SCCS provided its advice on the safe use of Butylated Hydroxytoluene and Acid Yellow 3 in cosmetics respectively. In view of SCCS's opinions, these two ingredients are newly included into the List of Restricted Ingredients with use restrictions.
Resorcinol (CAS No 108-46-3) is listed under Entry 22 of the restricted ingredients list. When used in hair dye products, products containing Resorcinol shall be labelled with "Do not use to dye eyelashes or eyebrows". Since hair product includes product intended for coloring eyebrows in accordance with the Cosmetics Regulation, the warning in current labelling requirement should therefore be amended.
The details of these amendments are shown in the table below (the text in red indicates changes to the previous list):
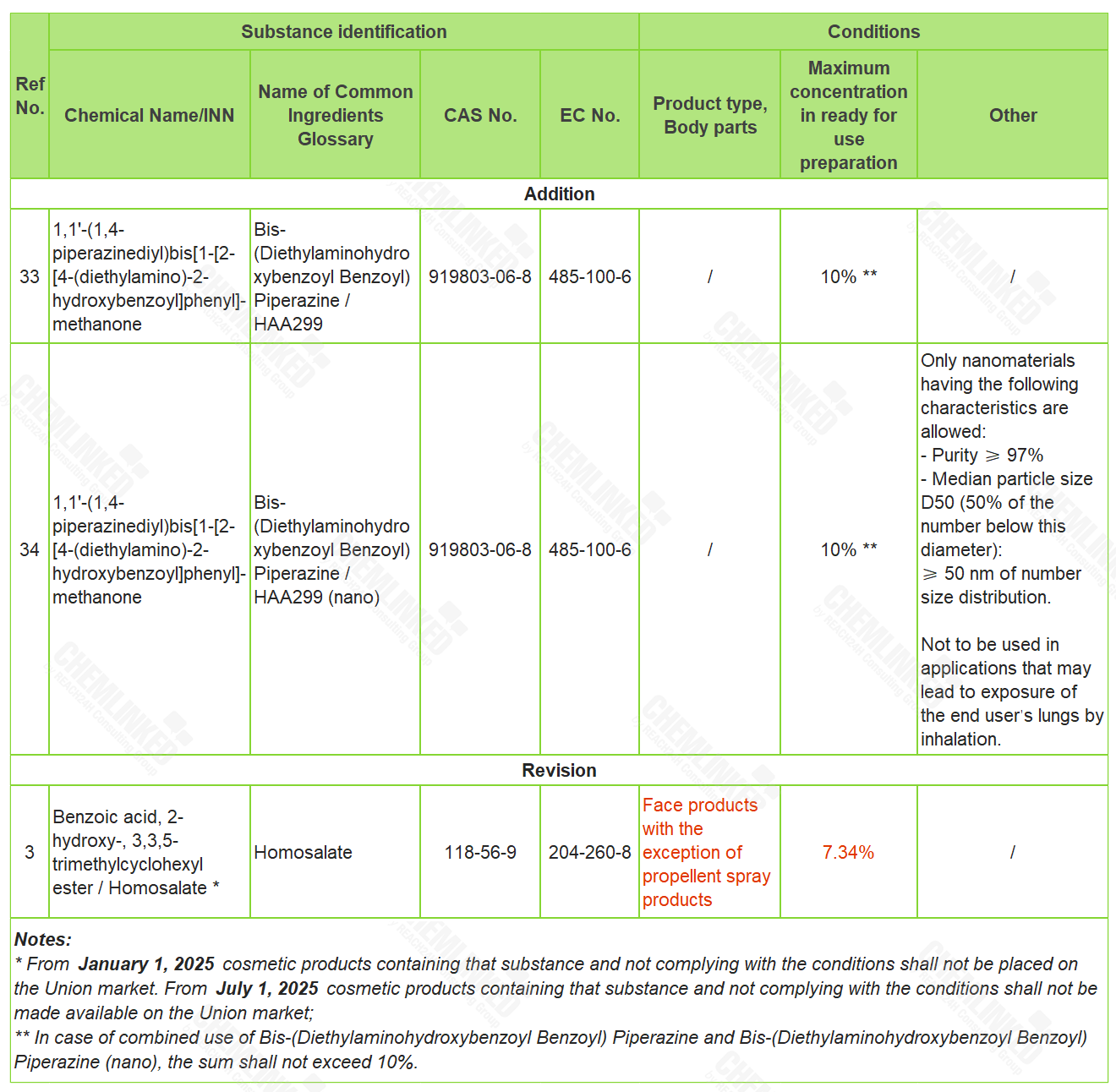
2. List of Permitted UV Filters: two added and one revised
HAA299 (CAS No 919803-06-8) is currently not regulated under the Cosmetics Regulation. Based on the data provided by the industry, SCCS released the opinion SCCS/1533/14 and SCCS/1634/21, and concluded that the use of HAA299 (in non-nano or nano form) as a UV-filter in cosmetics would result in a potential risk to human health when its concentration had exceeded a certain level. In light of SCCS's opinions, this ingredient should therefore be included into the Cosmetics Regulation to ensure its safe use in cosmetics.
Homosalate (CAS No 118-56-9) is a UV filter included in the List of Permitted UV Filters. As a response to industry's concern for its potential endocrine disrupting properties, SCCS carried out a safety assessment, whose result shows that the use in cosmetics at the concentrations currently allowed may pose a risk to human health. In accordance with SCCS's safety assessment result, the use requirement of Homosalate shall be amended accordingly.
The details of these amendments are shown in the table below:
Further Reading
- EU SCCS Finalizes the Scientific Advice on Triclocarban and Triclosan
- EU SCCS Publishes the Revised Opinion on Vitamin A
- EU to Extend the Individual Labelling Requirement for 56 Newly Identified Fragrance Allergens in Cosmetics
Reference Links
[1] Commission Regulation (EU) 2022/2195 of 10 November 2022 amending Regulation (EC) No 1223/2009








