공지/교육
법령
[Chemlinked] Cosmetics Regulation Focus: What Are the Impacts of the EU Chemicals Strategy for Sustainability?
첨부파일
등록일 2023-05-03
조회수 5360
- The introduction of the EU Chemicals Strategy for Sustainability has significant impacts on the Cosmetics Regulation.
- In line with the strategy’s objectives, the Cosmetics Regulation is likely to place bans on new hazard categories, extend the “Generic Approach to Risk Management”, improve the efficiency and coherence of safety assessments, take into consideration of chemicals’ combined effects in the risk assessment, consolidate the management of specific substances, and include digital labelling as a feasible labelling option.
Announced in December 2019, the European Green Deal (EGD) is the EU's new growth strategy to transform Europe into a sustainable and carbon-neutral economy by 2050. Specifically, it develops concrete strategies and action plans in eight policy areas, one of which is to tackle pollution from all sources and move towards a toxic-free environment. On October 14, 2020, the European Commission published the Chemicals Strategy for Sustainability (CSS) to achieve this ambitious environmental convention. In line with the EGD, the strategy strives to better protect human health and the environment while promoting the innovation for safe and sustainable chemicals.
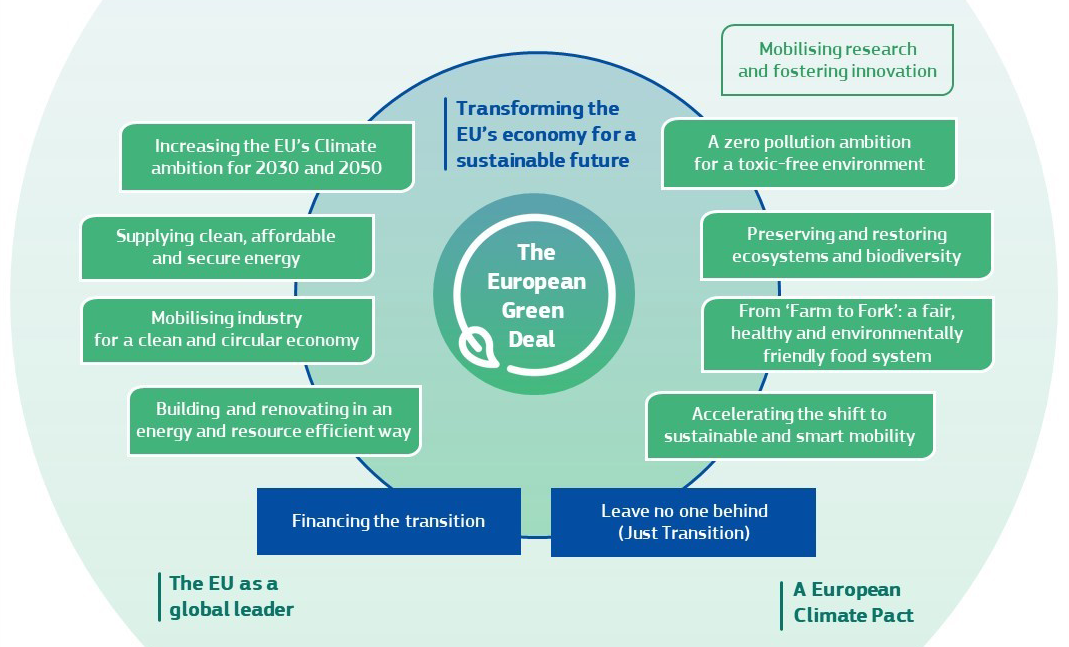
Eight Policy Areas of the European Green Deal
To achieve coherence within the EU's chemicals legislation, the CSS highlights the need to evolve the Regulation (EC) No 1907/2006 Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) and downstream legislations, including the targeted revision of Regulation (EC) No 1223/2009 (Cosmetics Regulation). For the revision of the Cosmetics Regulation, the Commission carried out an Inception Impact Assessment at the end of 2021, followed by a public consultation from March to June 2022. As the revision progresses, a legislative proposal is expected to be released in July.
As concluded in the Inception Impact Assessment, the following amendments will likely be introduced in the Cosmetics Regulations to reflect the CSS's objectives.
Placing Bans on New Hazard Categories
CMR substances are those classified as carcinogenic, mutagenic or toxic for reproduction. In line with REACH, the Cosmetics Regulation prohibits the use of CMR substances (those classified as carcinogenic, mutagenic or toxic for reproduction) in cosmetics, with minor exceptions under strict conditions.
In addition to CMR substances, there are other harmful chemicals that may pose similar risks to consumers and professional users. To comprehensively address these risks, the CSS proposes introducing endocrine disruptors, as well as persistent and mobile substances as new hazard categories to Regulation 1272/2008 on Classification, Labelling and Packaging of Substances and Mixture (CLP). In response, the Commission revised the CLP regulation, and formally introduced new hazard categories and criteria for the substances mentioned above on March 31, 2023.
Following the formal introduction of these new categories, substances in these categories will be identified as substances of very high concern (SVHCs) under REACH, and may be prohibited for use in cosmetics similar to the existing ban of CMR substances under the Cosmetics Regulation.
Extending the "Generic Approach to Risk Management"
A "generic approach to risk management" (GRA) is a preventive approach that triggers pre-determined risk management measures for chemicals based on their hazardous properties and exposure. In the past decades, with the GRA adopted for carcinogenic substances, they have been generally banned from most consumer products and for uses exposed to vulnerable groups, reducing citizens’ exposure to carcinogenic substances.
Currently, the vast majority of chemicals in the EU are regulated on a case-by-case basis and for each specific use. Responding to the ongoing health and environmental concerns for the most harmful chemicals, the CSS proposes to gradually extend the GRA to manage these substances, while allowing their use where proven essential for society.
In accordance with CSS's proposal, the Cosmetics Regulation is likely to extend the GRA from CMR substances to new hazard categories, to ensure that cosmetics do not contain most harmful substances. At the same time, the Cosmetics Regulation will review the criteria and processes to decide on exceptions to GRA bans, to bring them consistent with the essential use concept developed under the CSS.
Improving the Efficiency and Coherence of Safety Assessments
The EU's regulatory framework for chemical hazard and risk assessment and management is comprehensive and complex. Within this framework, chemical's safety assessments are being initiated under various pieces of legislation, and carried out by various EU agencies or scientific committees at different points in time. The complexity of these assessment procedures can lead to inconsistencies, slow procedures, inefficient use of resources and unnecessary burdens across the legislation, representing a specific challenge for authorities and stakeholders.
To address these issues, the CSS introduces the concept of "One Substance, One Assessment" to ensure that the initiation and priority setting of the safety assessments is done in a coordinated and transparent manner, and to build greater trust in the scientific underpinning of the EU decision-making process for chemicals. Specifically, the CSS proposes to establish an expert working group of Member States, Commission services and EU agencies to discuss initiatives on hazard/risk assessment on chemicals across chemical legislation, taking into account the specificities of each sector.
The Scientific Committee on Consumer Safety (SCCS) is an independent committee providing opinions on the health and safety risks of non-food consumer products. Regarding the safety of cosmetic ingredients and products, the SCCS opinions are recognized internationally, providing a basis for cosmetic ingredient regulations in ASEAN and Latin America. In line with the "One Substance, One Assessment" approach, the technical and scientific work on cosmetic ingredients performed by the SCCS may be reattributed to the European Chemicals Agency (ECHA).
Taking into Consideration of Chemicals' Combined Effects
People and other living organisms are exposed daily to a diverse range of chemicals originating from various sources. However, in the EU, the safety of chemicals is usually assessed by evaluating a single substance, or in some cases of mixtures intentionally added for particular uses. The combined exposure to multiple chemicals from different sources and over time is often overlooked.
To make up for this deficiency in risk assessment, the CSS considers it necessary to establish consistent legal requirements that consider the risks from simultaneous exposure to multiple chemicals across chemicals-related policy areas, and introduces the Mixture Assessment Factor (MAF) as an effective risk assessment tool.
Following CSS's initiative, the Cosmetics Regulation probably will introduce or reinforce provisions to take account of the combination effects in other relevant legislation, and introduce MAF to the safety assessment of cosmetic ingredients. Accordingly, The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation, the guideline for safety assessment, will be subject to revision.
Consolidating the Management of Specific Substances
Per- and polyfluoroalkyl substances (PFAS) are long-lasting chemicals which do not easily break down. They are widely used and proved to be a source of soil and water contamination due to their properties. In light of this, the CSS suggests that PFAS be phased out in the EU, unless it is proven essential for society. In cosmetics, PFAS compounds are sometimes used to condition and smooth the skin to make it appear shiny, or improve product spreadability. As evidence shows there are alternatives for using PFAS in cosmetics, the Cosmetics Regulation may also ban PFAS in the future.
In addition to PFAS, the CSS proposes to review the horizontal definition of nanomaterials. Individual definitions of nanomaterials existed in EU legislation in the food and cosmetics sector, while other EU laws already used the common definition from the Commission Recommendation 2011/696/EU. To achieve the consistency in regulatory interpretations and practices, the CSS considers it necessary to use coherent terminology to define nanomaterials. Guided by this proposal, the Commission released a new Recommendation in June 2022 to align legislation across all sectors with the revised definition. Consistent with this amendment, the Cosmetics Regulation will review the definition of nanomaterials. If the revised horizontal definition is adopted, more cosmetic ingredients will be considered as nanomaterials in the future. For cosmetics containing newly classified nanomaterials, they will also be subject to additional nano-notifications on the cosmetic products notification portal (CPNP).
Having Digital Labelling as a Feasible Labelling Option
With an aim to improve the communication of essential information on chemicals, the CSS considers it beneficial to simplify and digitalise the labelling requirements for chemicals. In particular, digital labelling could increase cost-efficiency for the industry, reduce administrative burdens by easing compliance with labelling requirements, and lead to simplified processes of compliance checks of products.
Currently, the labelling requirement for cosmetic products is limited to on-pack labelling, where the information shall be displayed on the container and/or the product's packaging. Considering the CSS's initiative, the Cosmetics Regulation will assess the use of digital tools for cosmetic labelling, and/or simply certain information on cosmetic labels.
출처 : Chemlinked








