법령정보
자료
[유럽, TBT통보문] Annex II(배합금지), Annex III(배합한도) 개정안 (스타이렌/아크릴레이트 등 나노물질)
첨부파일
등록일 2023-05-30
조회수 10225
2023년 05월 23일 European Commission에서는 EU 화장품 규정의 Annex II(배합금지) 및 Annex III(배합한도)에서 특정 나노물질을 추가하는 등의 내용으로 TBT 통보문을 발표하였습니다.
※ 개정 세부 내용은 아래 내용을 참고하시기 바랍니다.
출처 : Chemlinked
The EU is to ban the use of 12 nanomaterials, and restrict the use of Hydroxyapatite (nano) in cosmetics.
On May 23, 2023, EU notified WTO of a draft Commission Regulation which proposed to revise the prohibited and restricted ingredient lists in Regulation (EC) No 1223/2009 (Cosmetics Regulation). This draft measure was previously notified under EU/TBT/872, but is modified now to newly include the prohibition of Colloidal Silver (nano) and the restriction of Hydroxyapatite (nano). It is open for comments until July 22, 2023.1
The Cosmetics Regulation places particular emphasis on the safe use of cosmetics containing nanomaterials. If the European Commission has concerns regarding the safety of a nanomaterial, the Scientific Committee on Consumer Safety (SCCS) will be requested to give an opinion on its safety. In light of recent opinions from SCCS on nanomaterials, the Commission produced this draft.
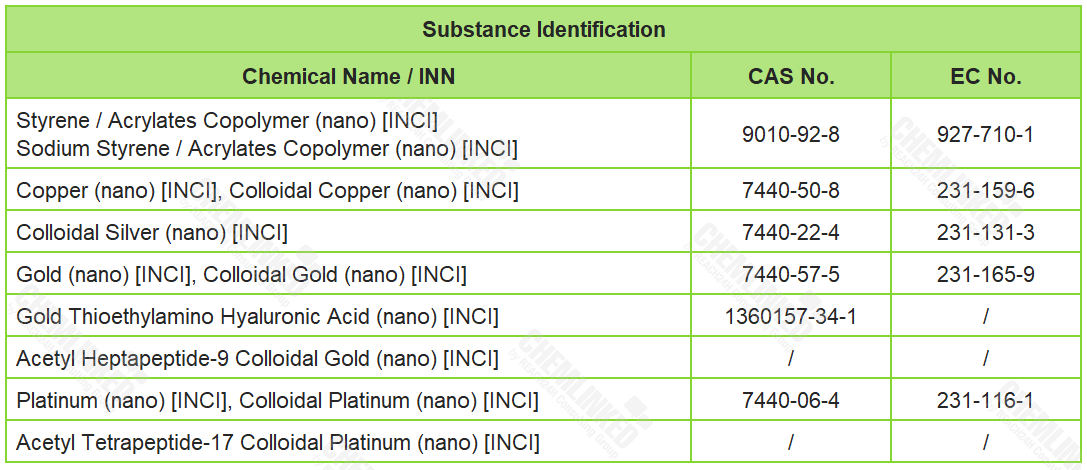
1. List of Prohibited Ingredients: 12 added
SCCS has conducted safety assessments on the nanomaterials listed in the table below, and concluded that currently available data is not sufficient enough to assess their safety in cosmetics. However, based on the available information in the scientific literature, SCCS indicates that there is a concern that these nanomaterials can pose a health risk to consumers when used in cosmetics. In response to SCCS's opinions, the draft proposes to ban the use of these nanomaterials in cosmetics.
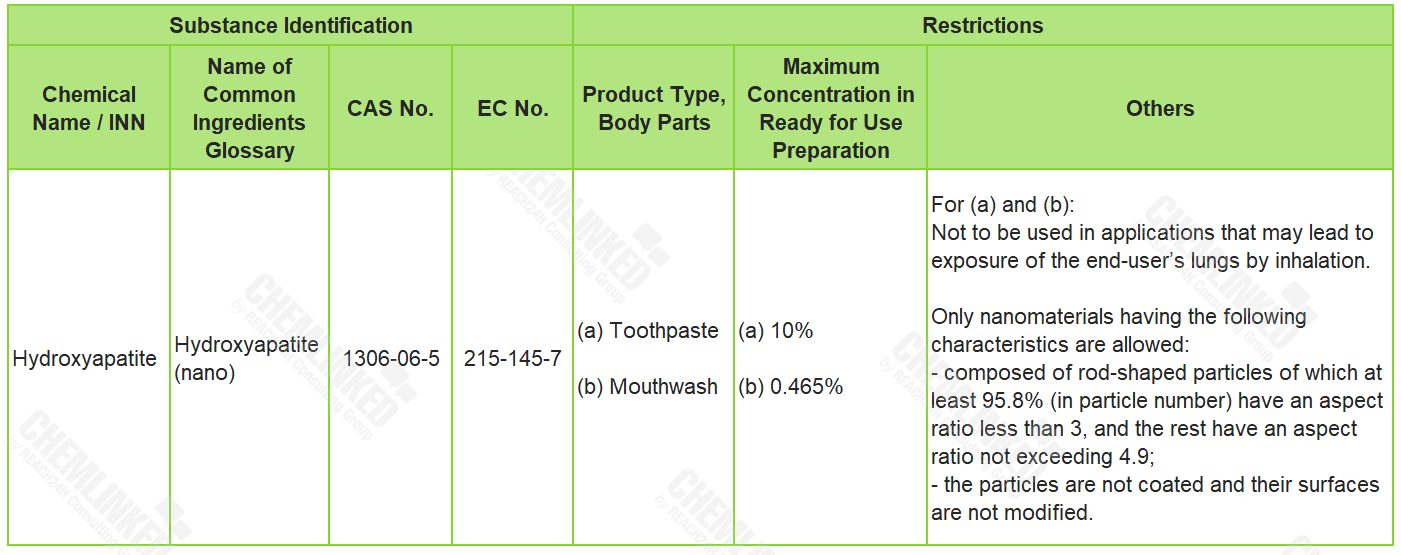
2. List of Restricted Ingredients: 1 added
Hydroxyapatite (nano) is currently not included in the ingredient lists in the Cosmetics Regulation. On March 22, 2023, SCCS adopted an opinion on it, concluding that it could be used safely in cosmetics under certain restrictions. In view of SCCS's opinion, it is newly included into the restricted ingredients list.
Further Reading
- EU Adopts the 12th Revision of The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation
- Cosmetics Regulation Focus: What Are the Impacts of the EU Chemicals Strategy for Sustainability?
- EU SCCS Releases Preliminary Opinion on Children's Exposure to Methyl Salicylate
Reference Links