법령정보
자료
[Chemlinked] Philippines Launches a New Round of Consultation on Amended Requirements for LTO Application
첨부파일
등록일 2023-06-12
조회수 5214
Compared with two previous drafts, the newly released amendment introduces stricter guidelines for initial, renewal and variation applications for the LTO, detailing the circumstances under which the LTO may be cancelled, and strengthens the requirements for the Qualified Person.
On June 5, 2023, Philippines Food and Drug Administration (FDA) released a new draft of Updated Guidelines on the Application for License to Operate of Health Product Establishments with the Food and Drug Administration (the Updated Guidelines). Any comments can be sent to pps@fda.gov.ph, with a copy to pfpid@fda.gov.ph, no later than June 30, 2023.1
In Philippines, companies that manufacture, import, distribute, or sell cosmetic products are required to obtain a License to Operate (LTO) from the FDA. In 2020, Administrative Order (AO) No. 2020-0017 was issued to introduce the required documents and procedures for applying for LTO. To further streamline the processes for initial, renewal, and variation applications for LTO, the FDA initiated consultations on amendments to AO No. 2020-0017 in May and October of last year. Compared with the previous two drafts, this new draft proposes the following adjustments related to cosmetics.
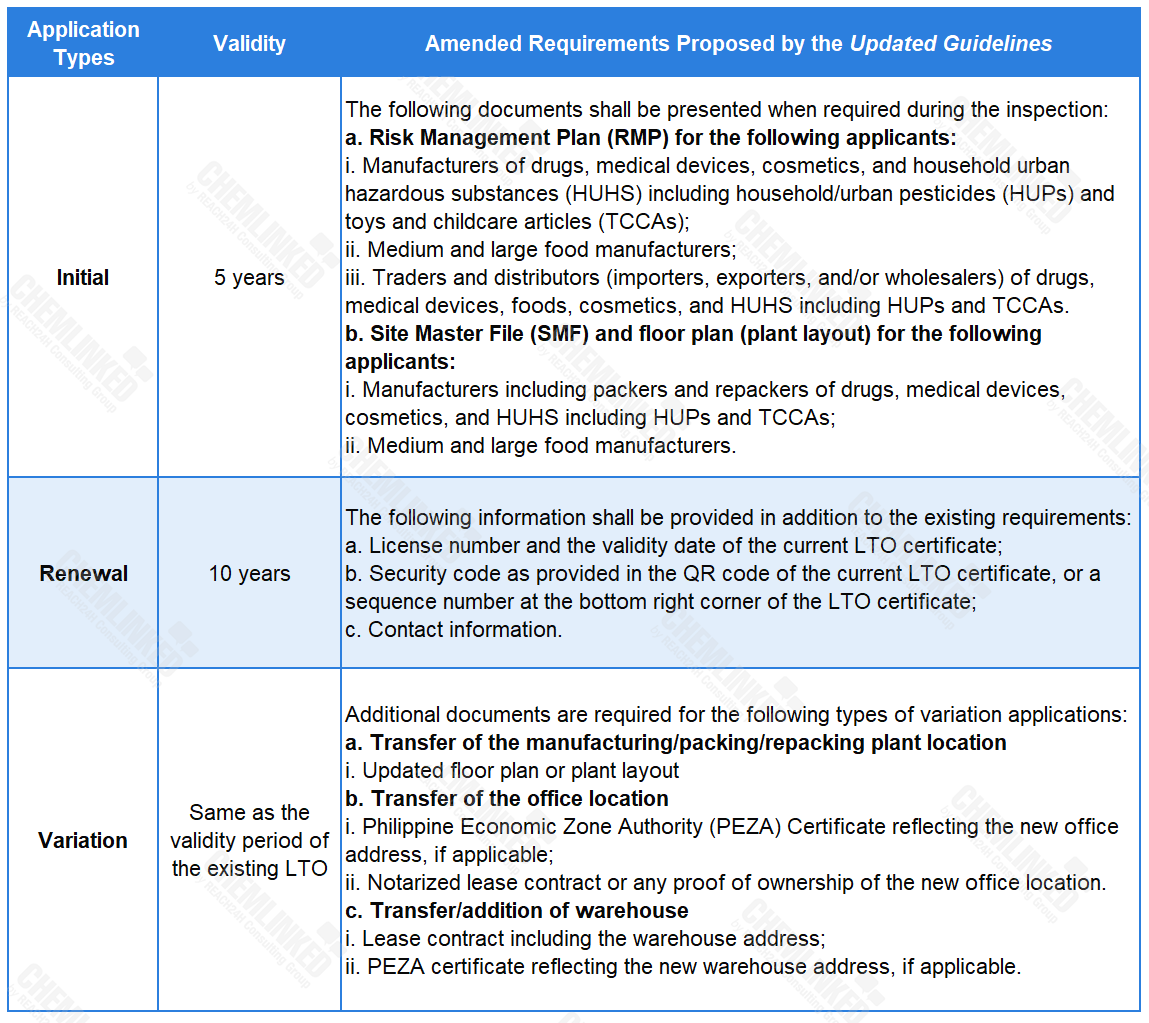
1. Introducing stricter guidelines for initial, renewal and variation applications for the LTO
Based on the current provisions concerning LTO applications, the Updated Guidelines supplements the requirements for different application types, and stipulates the corresponding validity period of the issued LTO.
2. Detailing the circumstances under which the LTO may be cancelled
As outlined in AO No. 2020-0017 and previous draft amendments, the LTO may be canceled due to, inspection verification, FDA-imposed penalty, voluntary filing, and the failure to file a renewal application. The Updated Guidelines supplements these provisions by describing additional instances that result in the cancellation of the LTO as follows:
- The application requirements submitted demonstrate that the company does not meet the required technical requirements or appropriate standards;
- The applicant has made misrepresentations or false entries, or has withheld any relevant data contrary to the provisions of relevant regulations;
- The owner has violated any of the terms and conditions of its license;
- There is non-compliance with any provision in the Updated Guidelines; and
- FDA determines that there are other similar grounds for cancellation.
When the LTO is cancelled, either through an inspection verification or voluntary surrender, the FDA shall retain jurisdiction over any violations committed by the companies while it was in operation.
3. Strengthening the requirements for the Qualified Person
A Qualified Person (QP) is an organic or full-time employee of the company who has the necessary technical expertise to oversee the company's activities related to health products. He/she is responsible for ensuring that the company meets the technical requirements set by the FDA. The previous two drafts imposed restrictions on the QP's employment, and specified the qualification required for the QP in cosmetic enterprises. On this basis, the Updated Guidelines now supplements that, in addition to the previously stated obligations, the QP shall:
- Ensure that all documentary and technical requirements for the LTO application are satisfied, and that the information provided is true and accurate based on existing records, legal documents, and other available information;
- Inform the FDA of any changes in the submitted documents and information, including the validity of his/her license, to ensure the consistent compliance with the FDA requirements.
Reference Links
출처 : Chemlinked