법령정보
자료
[Chemlinked] EU SCCS Finalizes the Opinion as to Salicylic Acid
첨부파일
등록일 2023-06-19
조회수 5443
On December 15, 2022, EU Scientific Committee on Consumer Safety (SCCS) initiated a two-month public consultation on the preliminary opinion on Salicylic Acid (CAS No. 69-72-7). Taking into account the feedback received, SCCS published the final opinion on June 9, 2023.1
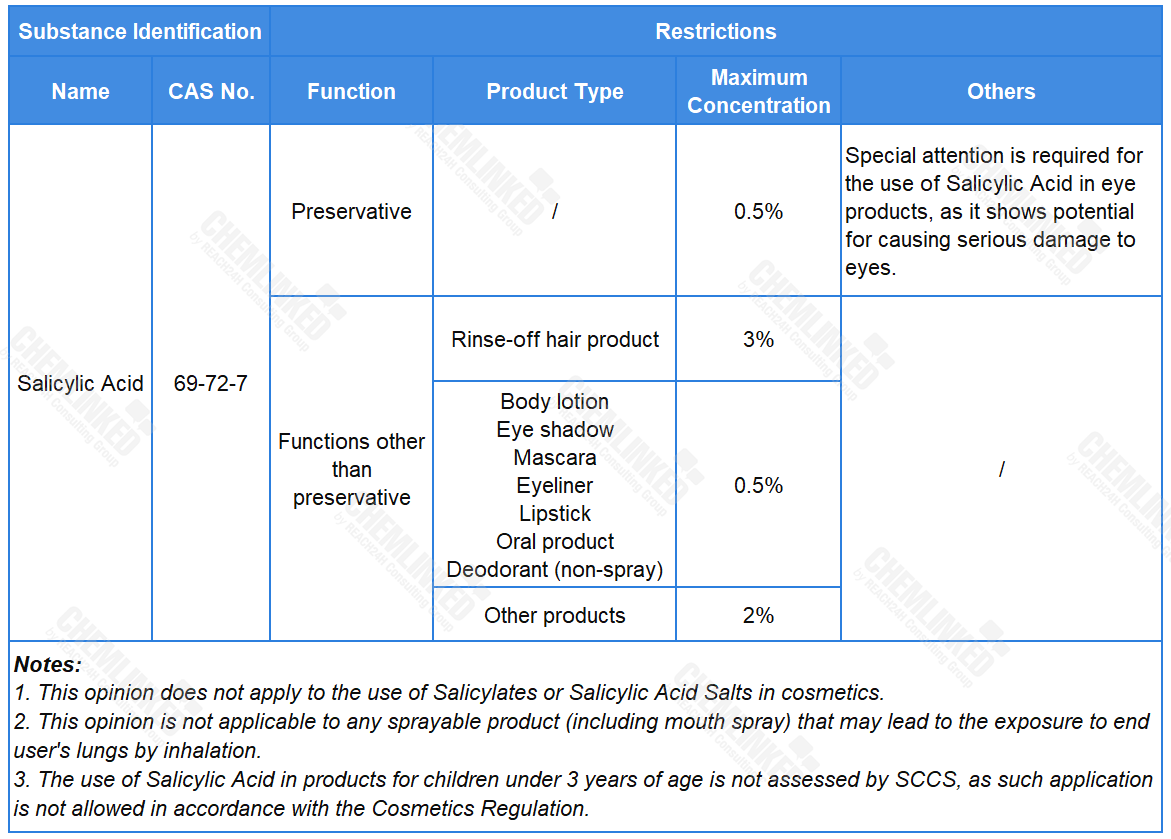
Salicylic Acid commonly serves as a denaturant, conditioning agent, exfoliant, anti-dandruff/anti-seborrheic agent, and preservative in cosmetics. Currently, its use is subject to restrictions laid in Regulation (EC) No 1223/2009 (Cosmetics Regulation). In response to concerns over its endocrine disrupting properties and potential use in oral products, the European Commission requested SCCS to carry out a safety assessment on Salicylic Acid.
In light of all available information, including the potential endocrine effects, SCCS establishes the safe use requirements for Salicylic Acid in cosmetics (see the table below). Compared to the current restrictions outlined in the Cosmetics Regulation, the final opinion now grants its use in oral products with specific maximum concentration.
Further Reading
- EU to Adopt 11 Changes to the Use Requirements for Cosmetic Ingredients
- EU to Amend the Use Requirements for 13 Nanomaterials in Cosmetics
- EU SCCS Consults on Opinions About Butylparaben and Methylparaben
Reference Links
[1] SCCS—Final Opinion on Salicylic Acid
출처 : Chemlinked