법령정보
자료
[유럽, TBT통보문] Annex II(배합금지), Annex III(배합한도), Annex V(보존제) 개정안 (알부틴 등)
등록일 2023-06-19
조회수 9736
2023년 06월 08일 European Commission에서는 EU 화장품 규정의 Annex II(배합금지) 및 Annex III(배합한도), Annex(보존제)의 물질을 개정하는 내용으로 TBT 통보문을 발표하였습니다.
※ 개정 세부 내용은 아래 내용을 참고하시기 바랍니다.
출처 : Chemlinked
The EU introduced 11 changes to the ingredient lists in Cosmetics Regulation, including:
1) Adding one prohibited ingredient, and eight restricted ingredients;
2) Amending the use requirements for two permitted preservatives.
On June 8, 2023, EU notified WTO of a draft regulation that aims at revising the ingredient lists in Regulation (EC) No 1223/2009 (Cosmetics Regulation). The draft proposes to prohibit and restrict the use of certain cosmetic ingredients respectively, following the opinions from the Scientific Committee on Consumer Safety (SCCS), and is open for comments until August 7, 2023.1
Detailed amendments to each list are as follows (the text in red indicates revisions to the previous list):
1. List of Prohibited Ingredients: one added
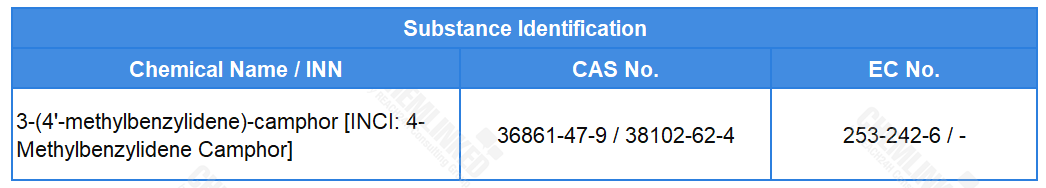
4-Methylbenzylidene Camphor (CAS No. 36861-47-9 / 38102-62-4) is currently regulated under the Cosmetics Regulation as a permitted UV-filter. As per opinion SCCS/1640/21, the use of this substance in cosmetics was potentially risky to human health. Therefore, the draft proposes to prohibit its use, and delete the corresponding entry from the list of permitted UV-filters.
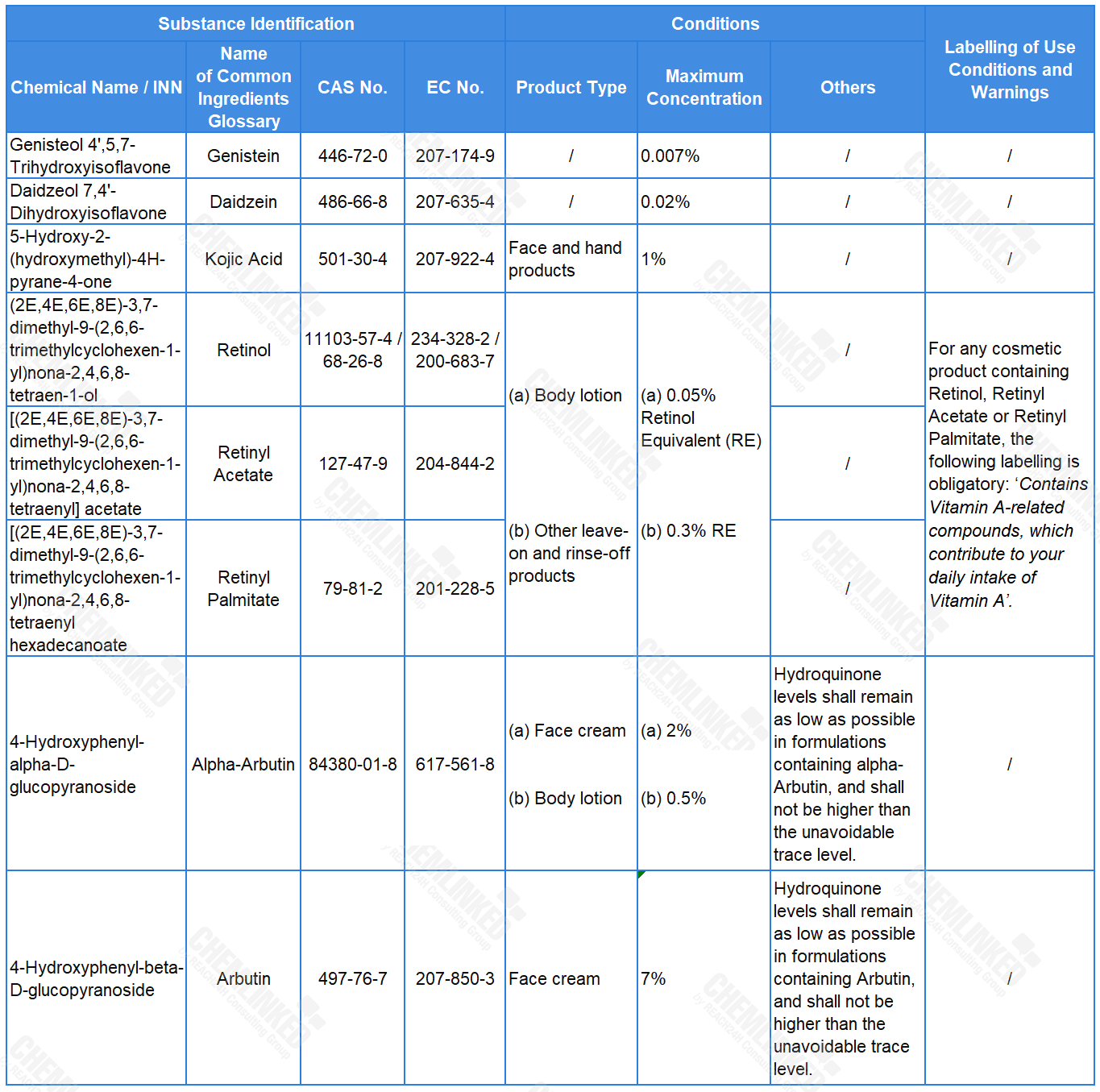
2. List of Restricted Ingredients: eight added
Currently, the ingredients listed in the table below are not subject to any use conditions under the Cosmetics Regulation. To guide their use in cosmetics and ensure the protection of human health, the European Commission mandated SCCS to carry out safety assessments on them. Based on SCCS's opinions, the draft proposes to establish restrictions on them in the restricted ingredients list.
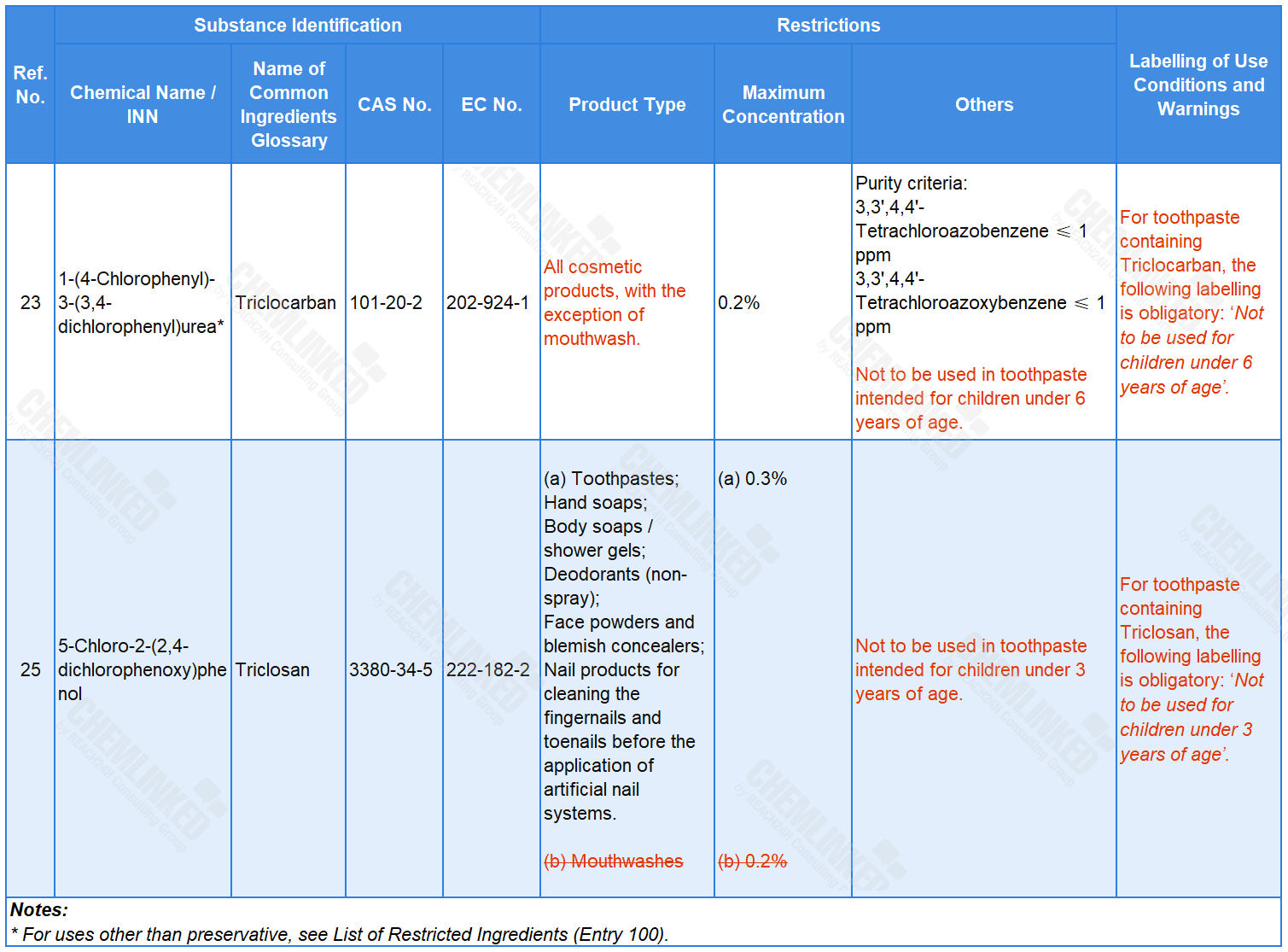
3. List of Permitted Preservatives: two revised
Triclosan and Triclocarban are two preservatives permitted for use in cosmetics. In response to concerns related to their potential endocrine disrupting properties, SCCS assessed their use in cosmetics and released its final advice in October 2022. According to SCCS, the use of Triclosan and Triclocarban in cosmetics may endanger human health under certain conditions. In light of this advice, the draft proposes to further restrict their use by revising their applicable product types, and introducing additional labelling requirements.
Further Reading
- New EU Regulation on General Product Safety to Take Effect in 2024
- EU Adopts the 12th Revision of The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation
- EU to Amend the Use Requirements for 13 Nanomaterials in Cosmetics
Reference Links