공지/교육
법령
[Chemlinked] EU SCCS Consults on Opinion as to Salicylic Acid
첨부파일
등록일 2022-12-19
조회수 15472
On December 15, 2022, EU Scientific Committee on Consumer Safety (SCCS) released the preliminary opinion on Salicylic Acid (CAS No. 69-72-7). The opinion is open for comments until February 17, 2023.1
Salicylic Acid is used in cosmetics commonly as denaturant, hair and skin conditioning agent, exfoliant, anti-sebum agent, anti-dandruff/anti-seborrheic agent, and product preservative. Currently, it is regulated under the restricted ingredients list and permitted preservatives list in Regulation (EC) No 1223/2009 (Cosmetics Regulation), with specific maximum concentrations and use conditions. In view of its endocrine disrupting properties, European Commission mandated SCCS to carry out a safety assessment for this substance.
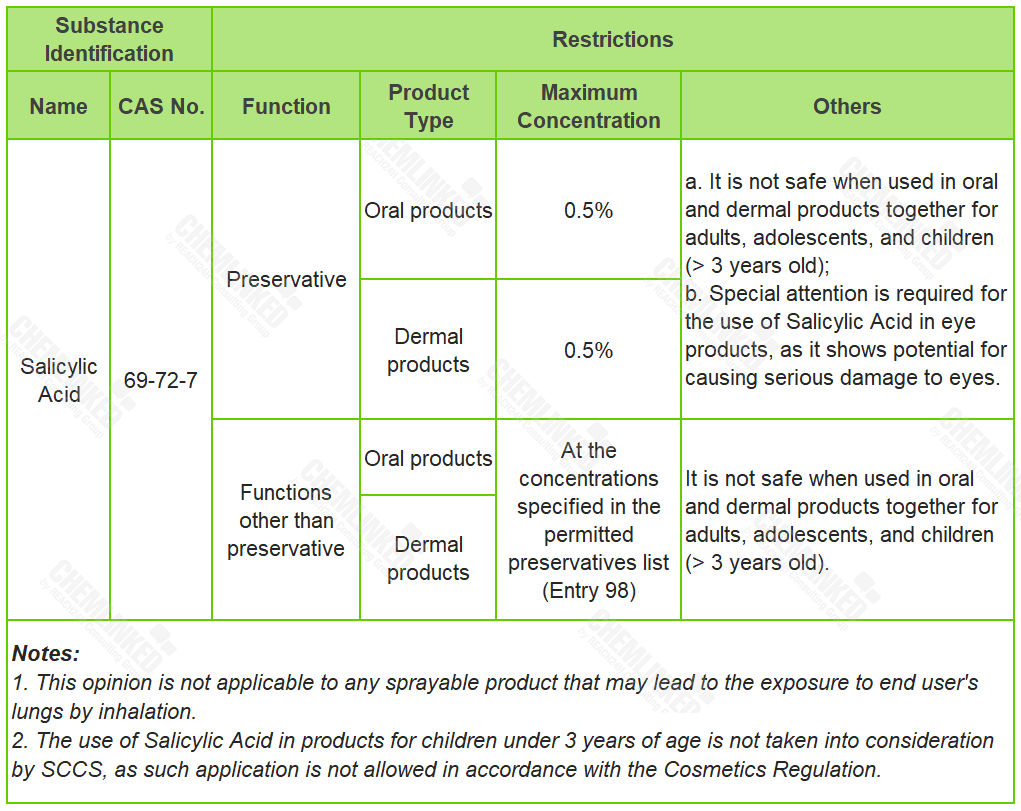
In consideration of all available information shown in the assessment, including the potential endocrine effects, SCCS concludes the safe use requirements for Salicylic Acid in cosmetics (see the table below). Compared to the restrictions laid in the Cosmetics Regulation, SCCS opinion allows the use of Salicylic Acid in oral products, and alerts the situation when it is used in oral and dermal products together.
Reference Links
[1] SCCS - Preliminary Opinion Open for Comments on Salicylic Acid
출처 : Chemlinked







