공지/교육
법령
[Chemlinked] ASEAN Amends Cosmetic Ingredient Annexes to ACD: 42 New Ingredients Included and 10 Ingredients Revised
첨부파일
등록일 2023-02-03
조회수 17810
The main amendments include:
1) adding 42 prohibited ingredients, and revising the requirements for 1 prohibited ingredient;
2) revising the requirements for 4 restricted ingredients;
3) revising the requirements for 1 permitted colorant;
4) revising the requirements for 3 permitted preservatives;
5) revising the requirements for 1 permitted UV-filter.
In January 2023, ASEAN introduced the latest amendments to the ingredient annexes to ASEAN Cosmetic Directive (ACD). These amendments were approved in the 36th ASEAN Cosmetic Scientific Body (ACSB) meeting, and will officially take effect on November 21, 2024.
Detailed amendments to each annex are as follows (the text in red indicates changes to the previous lists):
1. Annex II - List of Prohibited Ingredients: 42 added and 1 revised
The meeting agreed to newly include 42 prohibited ingredients, based on the amendments adopted by EU in Commission Regulation (EU) 2019/831, Commission Regulation (EU) 2019/1966, Commission Regulation (EU) 2021/850, and Commission Regulation (EU) 2021/1902 as part of the reference. Besides, Myanmar updated other member states with its ban on the ingredient 4-[(tetrahydro-2H-pyran-2-yl)oxy]phenol, consistent with the amendments adopted in the 35th ACSB meeting.
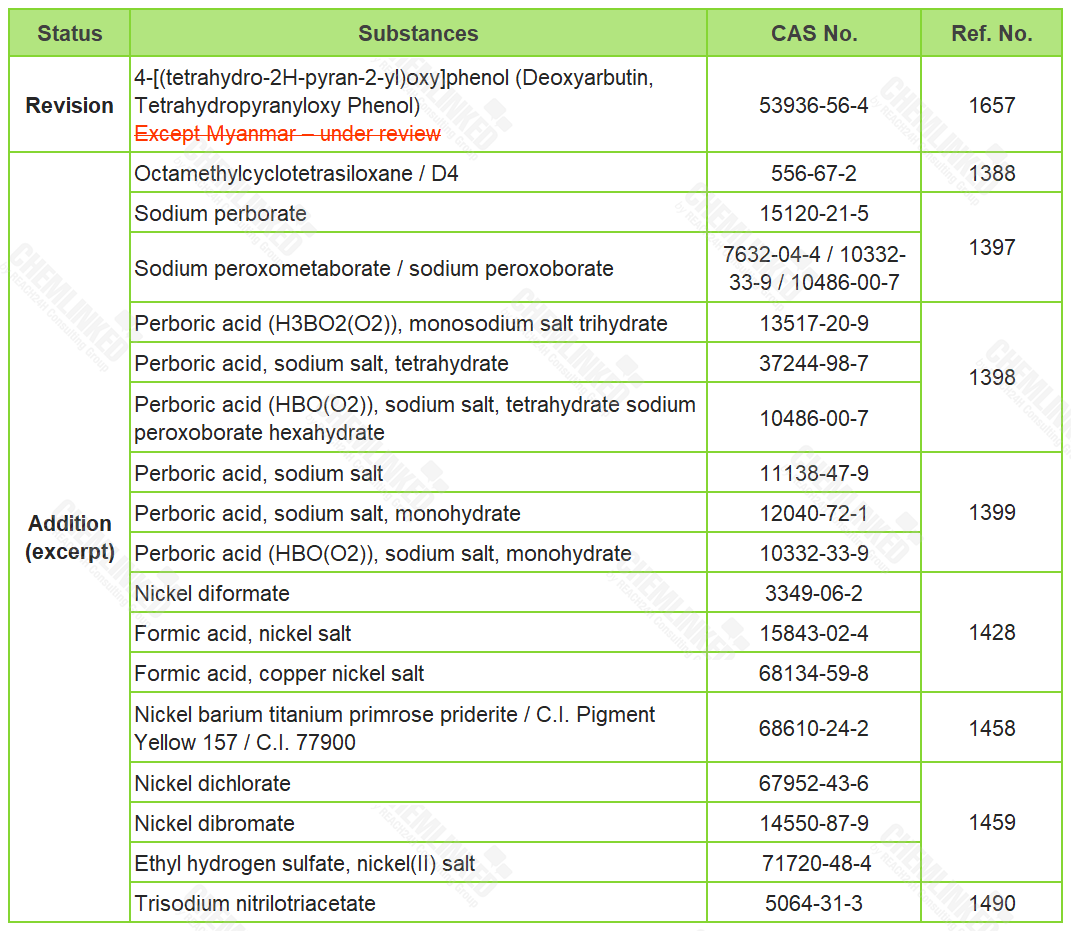 * Find the full list of 42 newly included prohibited ingredients here.
* Find the full list of 42 newly included prohibited ingredients here.
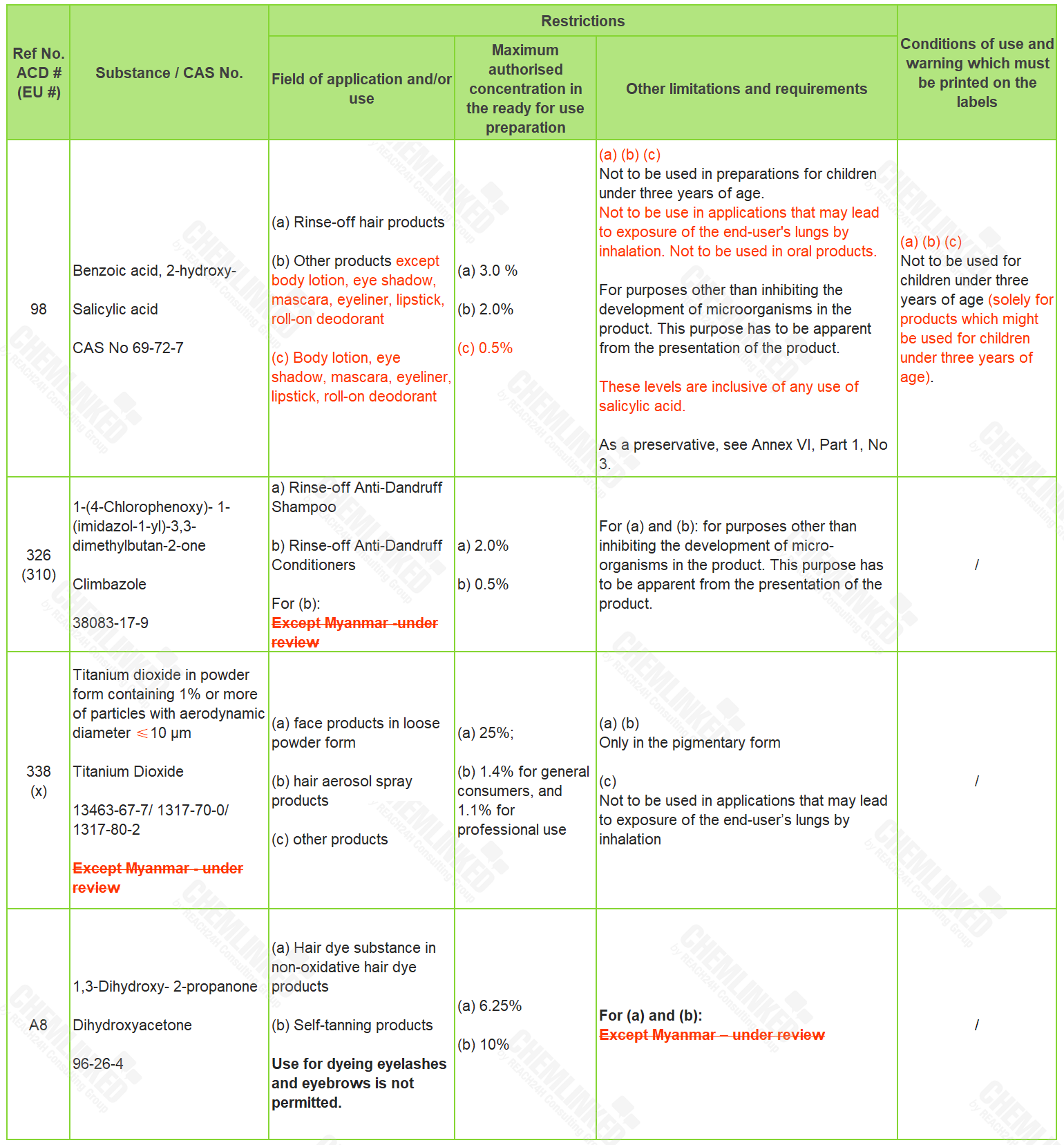
2. Annex III - List of Restricted Ingredients: four revised
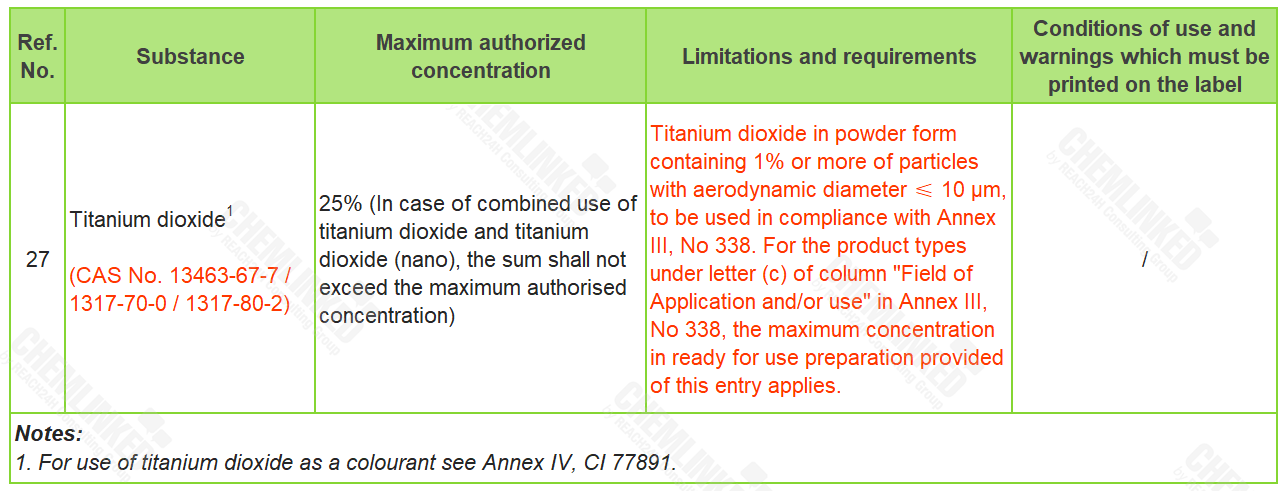
By reference to the latest use requirements for Salicylic Acid in EU Regulation (EC) No 1223/2009 (Cosmetics Regulation), the meeting agreed to amend the requirements for Salicylic Acid in Annex III to ACD. In addition, the meeting removed the limitation "Except for Myanmar" on ingredients Climbazole, Titanium Dioxide, and Dihydroxyacetone.
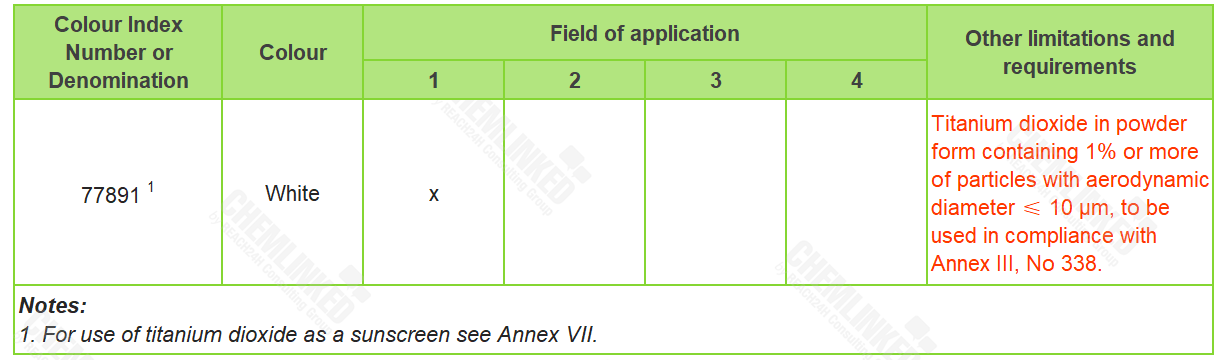
3. Annex IV - List of Permitted Colorants: one revised
In the meeting, all member states agreed to adopt EU's revision and amendment to the entry of Titanium Dioxide (CI 77891) in the Annex IV to ACD, except for purity criteria.
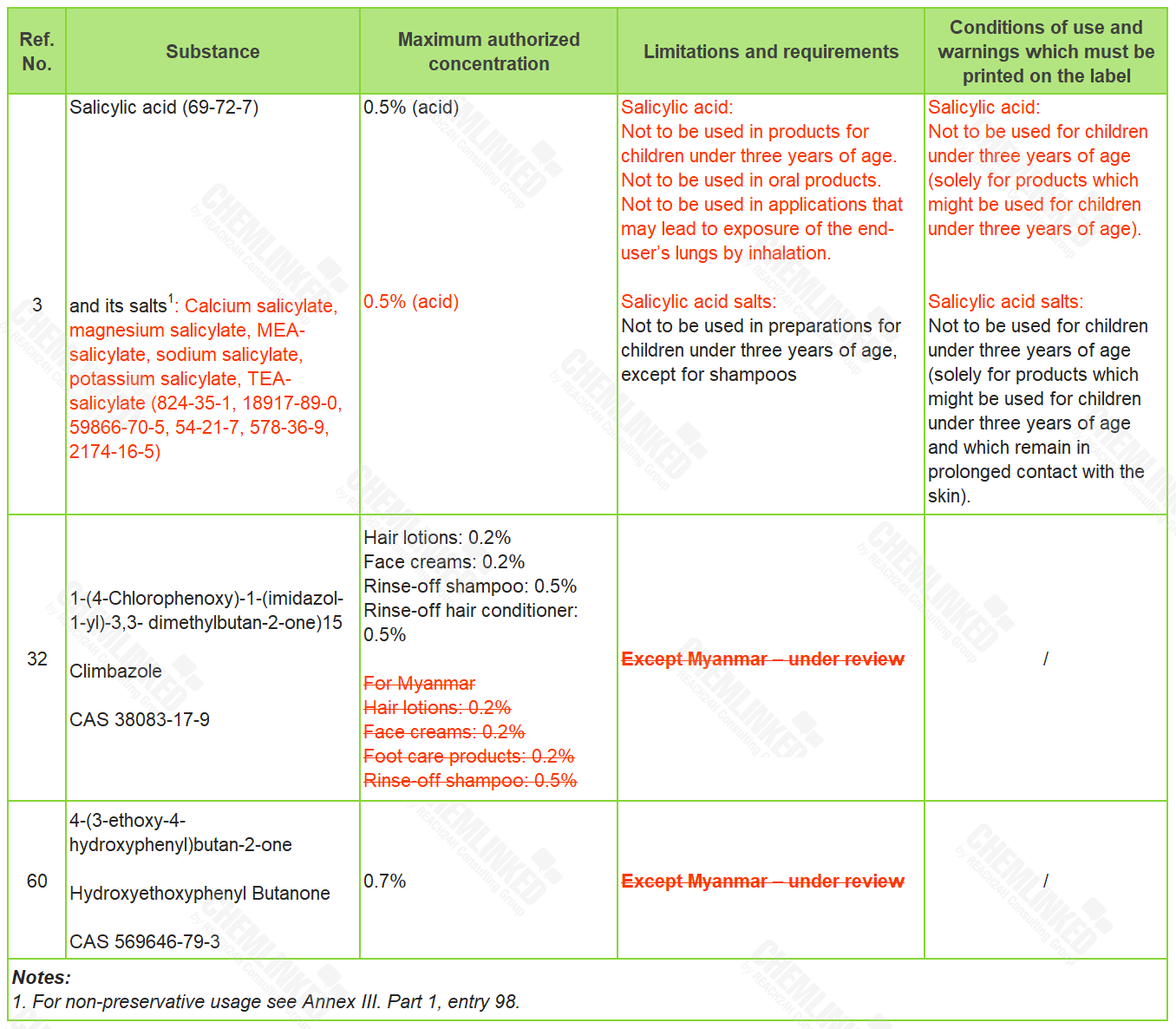
4. Annex VI - List of Permitted Preservatives: three revised
In terms of permitted preservatives, the meeting supplemented the identification and use requirements for Salicylic Acid. Besides, after this meeting, the latest use requirements for ingredients Climbazole and Hydroxyethoxyphenyl Butanone started to apply to Myanmar.
5. Annex VII - List of Permitted UV Filters: one revised
- addition to the above amendment to Annex IV, the meeting also updated the requirements for Titanium Dioxide when it is used as a UV filter in cosmetics.
출처 : Chemlinked







