공지/교육
법령
[Chemlinked] EU Safety Gate Alerts 212 Batches of Non-compliant Cosmetics in 2022
첨부파일
등록일 2023-03-09
조회수 15592
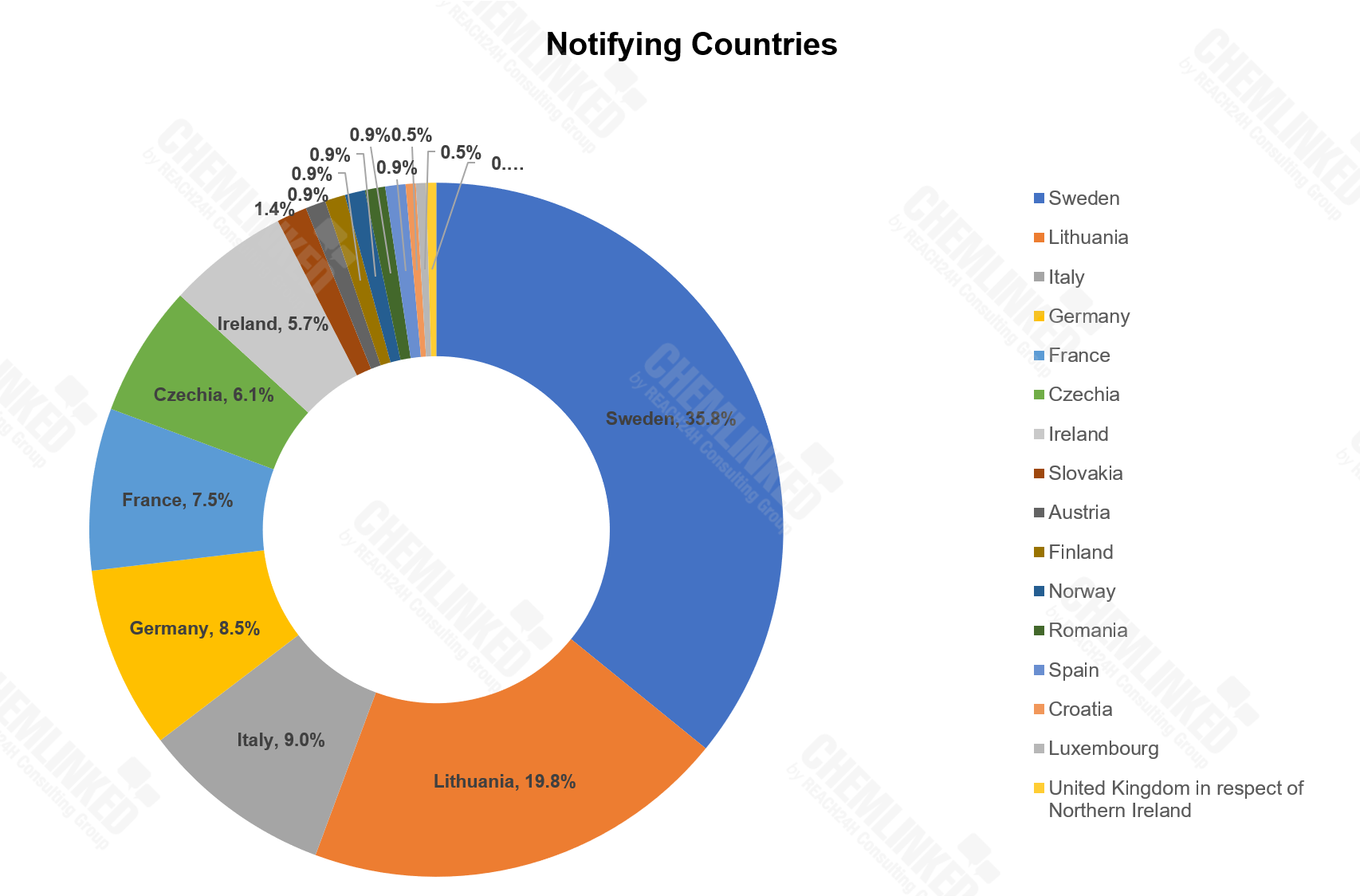
1) Most of the non-compliant cosmetics were notified by Sweden, Lithuania, Italy, Germany and France.
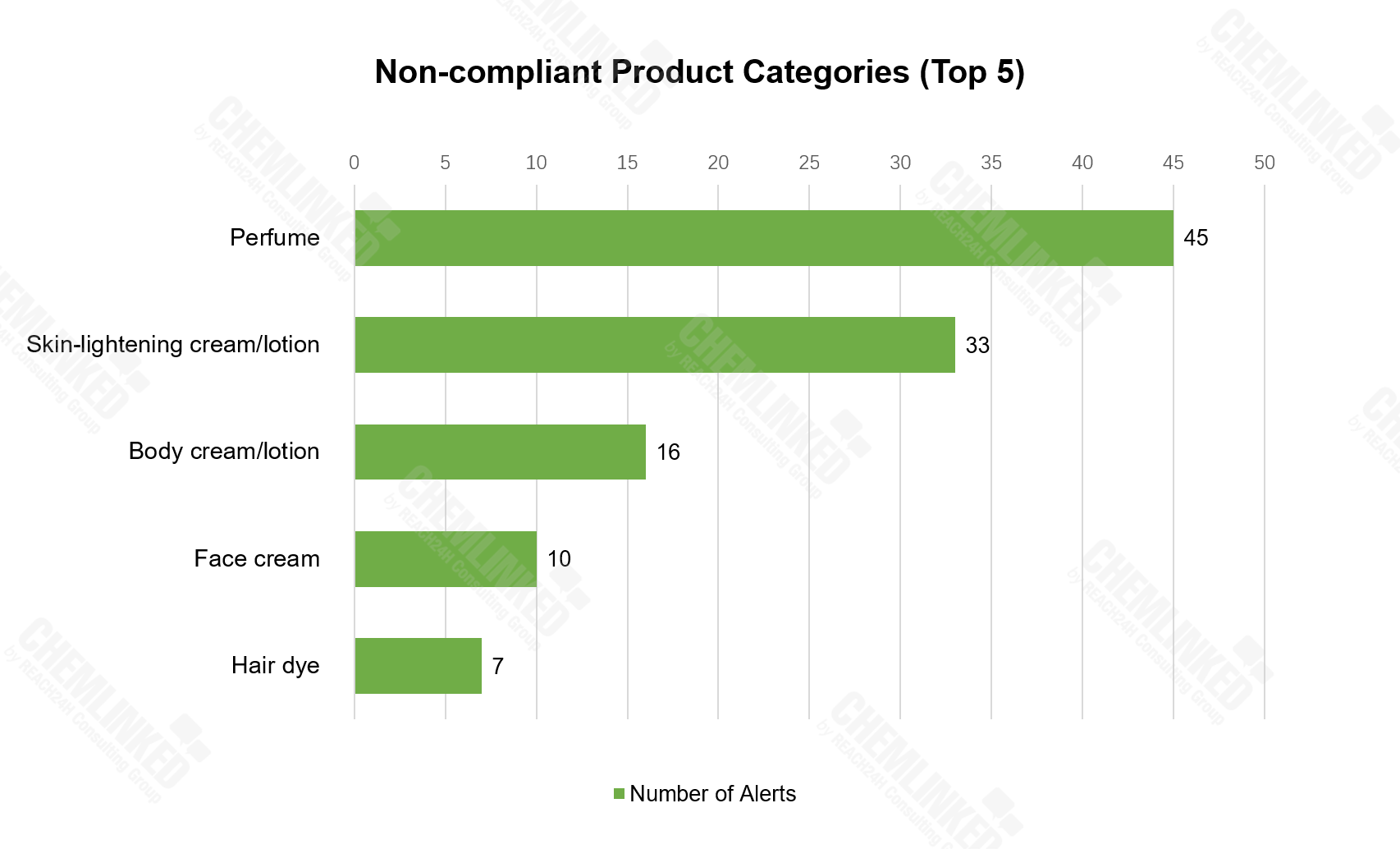
2) Perfume tops the list of non-compliance cosmetics, followed by skin-lightening cream/lotion, body cream/lotion, face cream and hair dye.
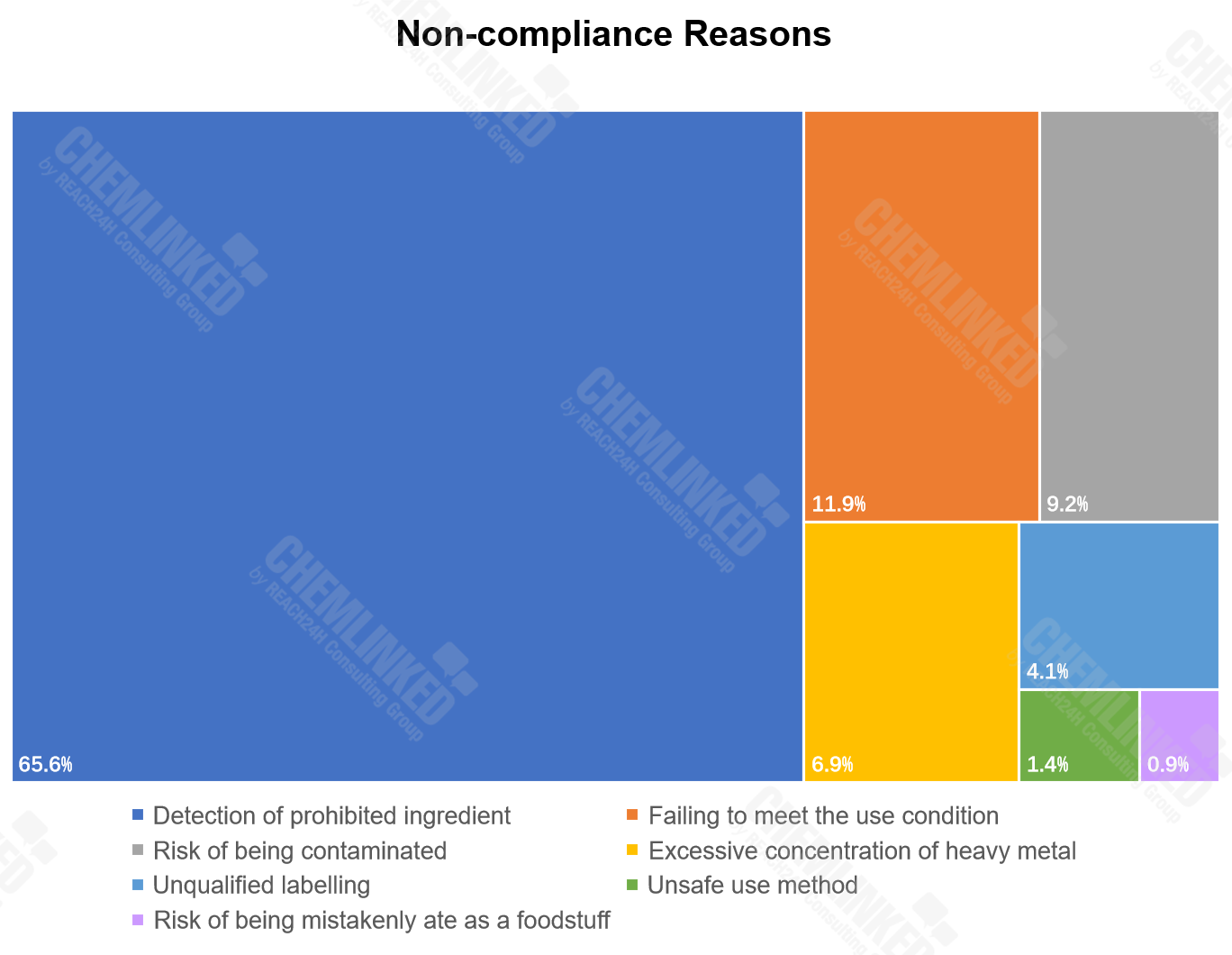
3) The main non-compliance reasons for the notified cosmetics include detection of prohibited ingredient, failing to meet the use condition, risk of being contaminated, and excessive concentration of heavy metal.
EU Safety Gate, formerly known as RAPEX, is a Europe-wide rapid alert system for dangerous non-food products. It enables national authorities in 30 countries (EU countries plus Iceland, Liechtenstein and Norway) to quickly exchange information and measures taken against dangerous products, and thus makes member countries possible to screen their markets and take appropriate action should the same product be found.
In 2022, EU Safety Gate published 212 alerts on non-compliant cosmetics notified by 16 countries, among which Sweden, Lithuania, Italy, Germany and France ranked the Top 5.
In terms of product category, perfume (21.1%) tops the list of non-compliant cosmetics, followed by skin-lightening cream/lotion (15.6%), body cream/lotion (7.5%), face cream (4.7%) and hair dye (3.3%).
For the notified cosmetics, the detection of prohibited ingredients (65.6%) is the primary non-compliance reason. Other major reasons include failing to meet the use condition (11.9%), risk of being contaminated (9.2%), excessive concentration of heavy metal (6.9%) and unqualified labelling (4.1%).
Noteworthy Compliance Requirements for the Above-mentioned Notified Cosmetics
1. Ingredient
In 2022, there were 143 alerts for cosmetics containing prohibited ingredients. The detected ingredients in these alerts include:
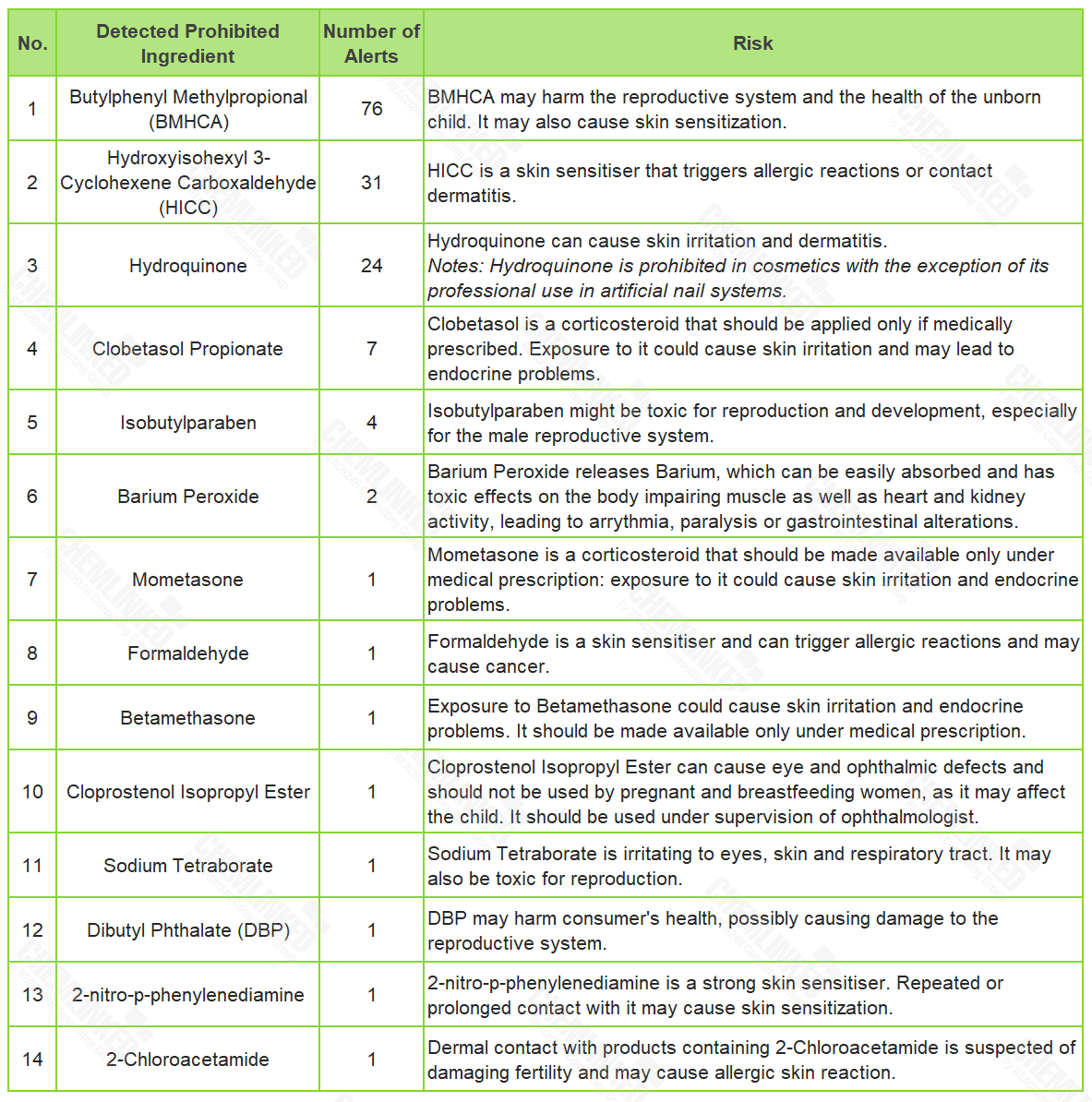
2. Use Condition
Among cosmetics notified in 2022, 11.9% of them failed to meet the use conditions established in Regulation (EC) No 1223/2009 (Cosmetics Regulation). ChemLinked has sorted out the ingredients involved in these non-compliance cases, and detailed their corresponding use conditions in the table below:
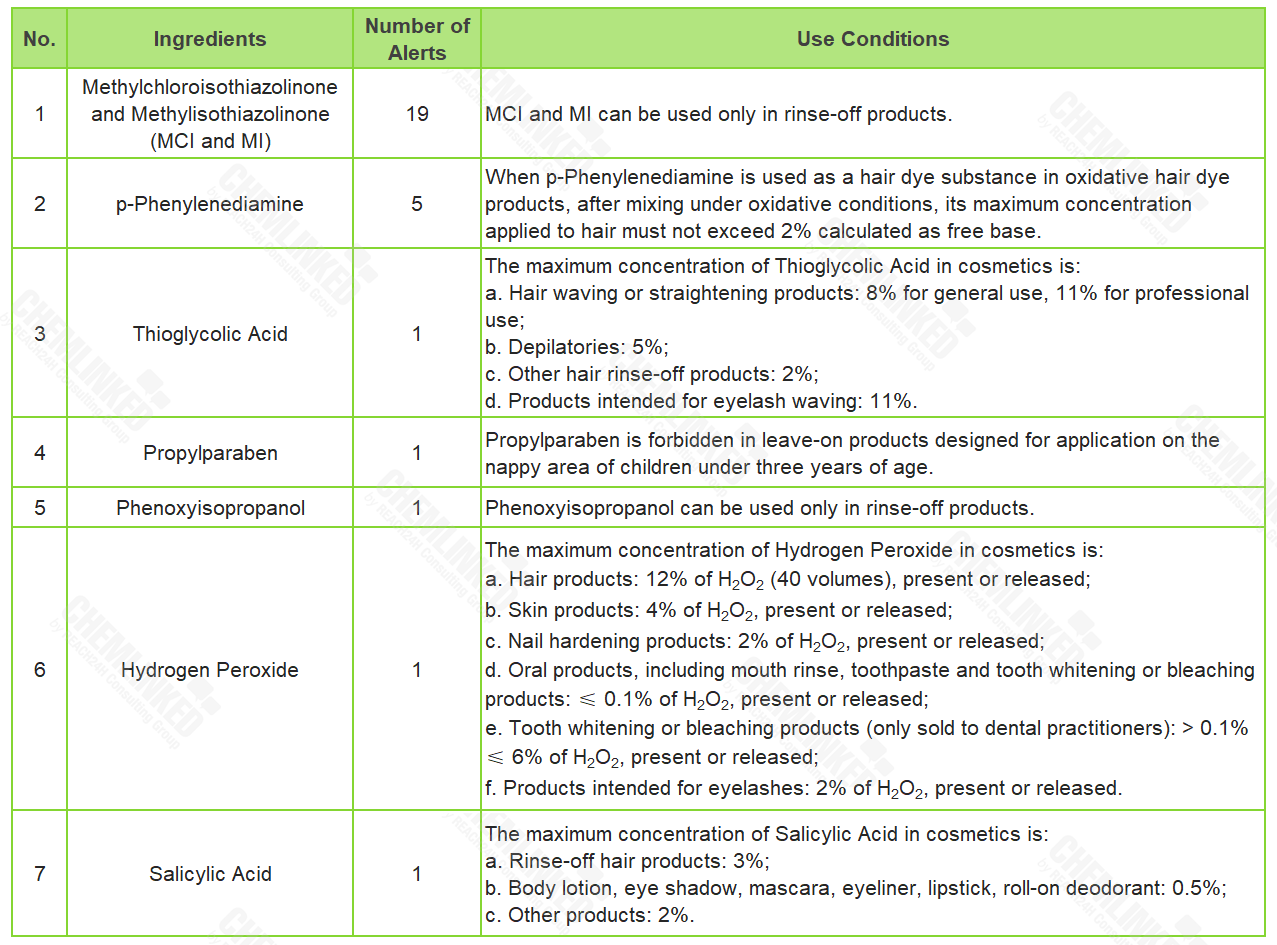
3. Unqualified Labelling
Subject to Cosmetics Regulation, cosmetics available on the EU market shall visibly bear a list of ingredients on its packaging, in descending order of the ingredients' weight at the time they are added to the product. Ingredients with concentrations of less than 1% can be listed in any order after those with concentrations of more than 1%.
Under this requirement, special attention shall be given to perfume and aromatic compositions as well as their raw materials. Rather than their common names, the compositions are required to be indicated with specific terms "Parfum" or "Aroma" in the list. On this basis, Cosmetics Regulation requires that, for identified fragrance allergens, their presence shall be individually indicated in the list in addition to terms "Parfum" or "Aroma" when their concentration exceeds 0.001% in leave-on products or 0.01% in rinse-off products.
4. Microbiological Specification
Exposure to microbiologically contaminated products may pose a risk of skin infection, in particular for sensitive consumers. At present, the European Standard EN ISO 17561:2014 is widely used by the cosmetics industry as a standard of microbiological limits. According to it, the microbiological quality of the finished cosmetic product needs to meet the specifications below:
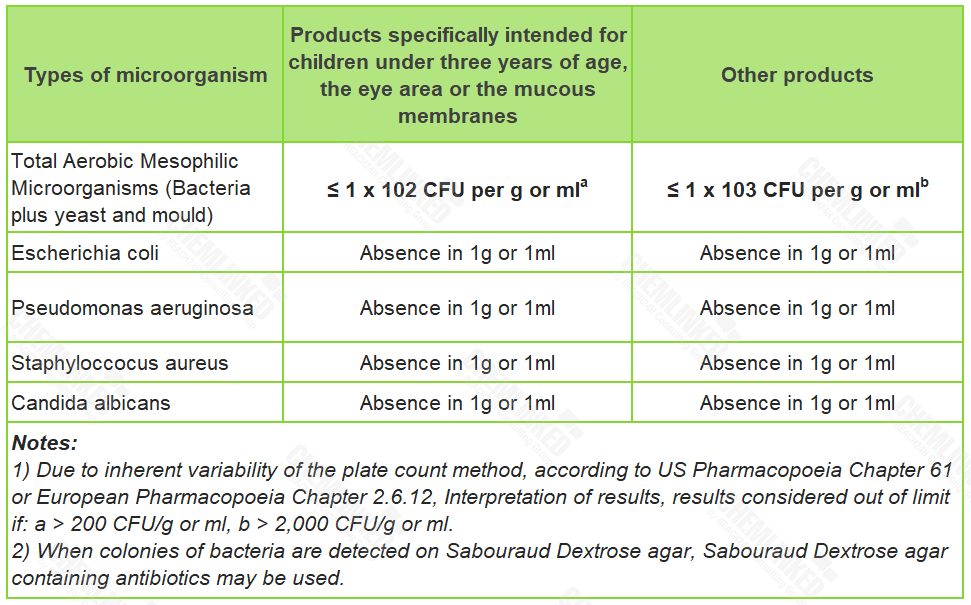
5. Heavy Metal Specification
Heavy metals are banned in cosmetics under Cosmetics Regulation, while traces are allowed if the concentration level will not pose a risk to human health. However, Cosmetics Regulation does not stipulate the specific limits for trace concentration. As a source of reference, the Member State Germany sets the limits (mg/kg) of unavoidable metal content in cosmetics, respectively:
- cadmium: 0.1;
- mercury: 0.1;
- antimony: 0.5;
- arsenic: 0.5 (or 2.5 for theatre make-up); and
- lead: 2.0 (or 5.0 for certain make-up products) and 0.5 (toothpaste).
Further Reading
- A Collection of SCCS Final Opinions and Scientific Advice in 2022
- EU SCCS Finalizes Opinion on Aluminium
- EU SCCS Consults on Opinion as to Hydroxyapatite (nano)
- EU SCCS Releases the Final Opinion on Alpha-arbutin and Beta-arbutin
출처 : Chemlinked







